
This process would also increase the percentage of lead oxide in the material. This is produced from PbO by roasting in a flow of air. Red lead (Pb 3O 4) can also be added to the PbO formed by these methods, as it is more conductive. Ball milling: Pieces of lead are put into a rotary mechanical mill, forming fine lead flakes, which are then oxidised in air and removed.Each droplet reacts with the air to form an oxide layer, giving 70 – 85% lead oxide. Barton pot: A fine stream of molten lead is inserted into a heated vessel.The lead can be oxidised by two processes: The Barton pot and the ball mill.
Grids can also be formed by mechanical working, either by cutting deep grooves into a sheet of steel, or by rolling up crimped strips and inserting them into holes in a cast plate, see Introduction to Deformation Processes TLP. The molds are closed and filled with sufficient molten lead to fill the mold, leaving some excess to form a sprue, which is then removed by cutting or stamping. Permanent steel molds are made from blocks by machining. “Book mold” casting is the most common method of production for the grid. The design is a simple grid framework with a “tab” or “lug” for connection to the terminal post. The function of the grid is to hold the active material and to conduct electricity between the active material and the battery terminals. A typical alloy would be 0.03 – 0.10% calcium and 0.5 – 1.0% tin (to enhance mechanical and corrosion properties). This is often used for telephone applications, and for no maintenance automotive batteries, since a more stable battery is required.
(2) Alkaline earth metals such as calcium can be used to stiffen the lead. These act as grain refiners, decreasing the grain size of the lead and thereby increasing its hardness and strength. (1) Using below 4% the battery water consumption is reduced, however it is then necessary to add small amounts of other elements such as sulphur, copper, arsenic and selenium. There are two possible solutions to this problem: This means that the water consumption in the cell increases and frequent maintenance is necessary. However, during the operation of the battery the antinomy dissolves and migrates to the anode where it alters the cell voltage. Pure lead is too soft to use as a grid material so in general the lead is hardened by the addition of 4 – 6% antimony. Under certain circumstances the lead sulphate products at both the electrodes achieve an irreversible state, making the recharging process very difficult. Sealed batteries are made safer by allowing the gases to recombine within the cell. Overall: Pb + PbO 2 +2H 2SO 4 → 2PbSO 4 + 2H 2Oĭuring the charging process, the reactions at each electrode are reversed the anode becomes the cathode and the cathode becomes the anode.ĭuring charging, given the high voltage, water is dissociated at the two electrodes, and gaseous hydrogen and oxygen products are readily formed leading to the loss of the electrolyte and a potentially explosive situation.

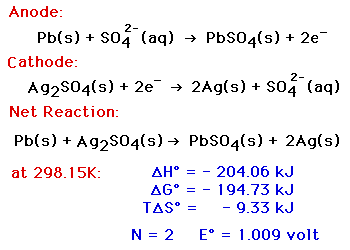
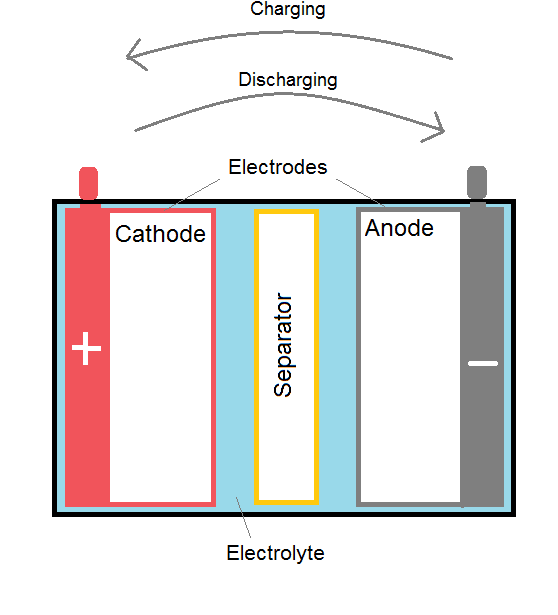
The following half-cell reactions take place inside the cell during discharge:Īt the anode: Pb + HSO 4 – → PbSO 4 + H + + 2e –Īt the cathode: PbO 2 + 3H + + HSO 4 – + 2e – → PbSO 4 + 2H 2O The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. Best performance with intermittent discharge. The electrodes were also changed to a tubular design.Ĭharacteristics in brief (for an SLI battery)ĭischarge characteristics: Generally quite curved, particularly at higher discharge rate. The cell was further developed by initially coating the lead with oxides, then by forming plates of lead oxide by coating an oxide paste onto grids. Strips of lead foil with coarse cloth in between were rolled into a spiral and immersed in a 10% solution of sulphuric acid. It was first developed in 1860 by Raymond Gaston Planté. The most common is the SLI battery used for motor vehicles for engine Starting, vehicle Lighting and engine Ignition, however it has many other applications (such as communications devices, emergency lighting systems and power tools) due to its cheapness and good performance. The lead acid battery is the most used secondary battery in the world.


 0 kommentar(er)
0 kommentar(er)
